A recent study published by Rupert W Leong, Vipul Jairath, and Christopher Ma breaks new ground in understanding why many patients with inflammatory bowel disease (IBD) continue to experience gastrointestinal symptoms even after their disease appears to be under control as shown via endoscopy. The comprehensive research, titled “Intestinal permeability accounting for ongoing gastrointestinal symptoms following endoscopic remission in IBD,” delves into the concept of intestinal permeability—often described as a leaky gut—as a potential factor contributing to persistent symptoms in patients who clinically are in remission.
Inflammatory bowel disease, which includes Crohn’s disease and ulcerative colitis, affects millions worldwide and poses significant management challenges due to its chronic and relapsing nature. Standard treatment goals typically aim to induce and maintain clinical and endoscopic remission. However, despite achieving what looks like healing of the intestinal lining under an endoscope, many patients report continuous discomfort and impaired quality of life due to ongoing symptoms like diarrhea and abdominal pain.
This pioneering research provides evidence suggesting that even when endoscopic images indicate remission, abnormal intestinal permeability may still persist and play a crucial role in the continuance of symptoms. This finding could shift current therapeutic strategies and suggest new treatment targets that more directly address this underlying issue, aiming for a more comprehensive approach to symptom alleviation and patient care in IBD.
The groundbreaking study by Rupert W Leong, Vipul Jairath, and Christopher Ma emerges against a backdrop of increasing recognition of the intricacies associated with inflammatory bowel disease (IBD) treatment. IBD, encompassing both Crohn’s disease and ulcerative colitis, has traditionally been viewed through the lens of visible inflammation and damage to the intestinal tract. Methods such as endoscopy, which allows direct visualization of the intestinal mucosa, have been crucial for diagnosing and evaluating the severity of the disease, as well as the response to treatment. Achieving endoscopic remission—where no signs of active inflammation are detectable—has long been a primary target for clinicians aiming to control the disease’s progression and improve patient outcomes.
However, clinical practice has noted a puzzling discrepancy where patients continue to suffer from significant symptoms such as abdominal pain, diarrhea, and fatigue even when endoscopic examinations suggest that the disease is well-controlled or in remission. This discrepancy poses a significant challenge, as it complicates the management of IBD and affects patients’ quality of life. It has led clinicians and researchers to speculate that other, less visible disease mechanisms might contribute to the symptomatology seen in IBD.
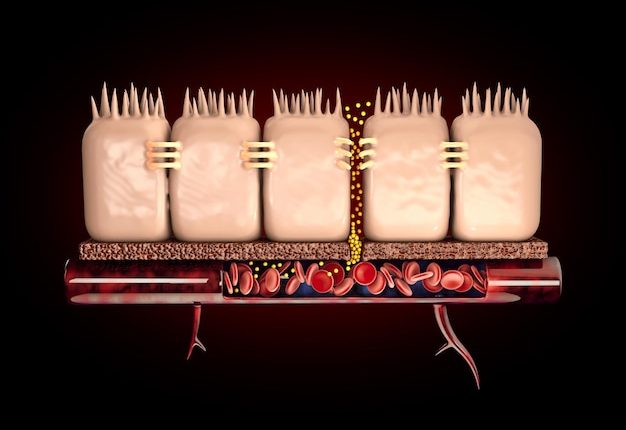
Intestinal permeability, or leaky gut syndrome, refers to the condition where the integrity of the intestinal barrier is compromised, allowing the translocation of bacteria, toxins, and antigens from the gut lumen into the mucosa and potentially into the systemic circulation. This phenomenon can trigger inflammatory and immune responses, which may contribute to ongoing symptoms in the absence of overt inflammation visualized during endoscopic exams.
Previous studies have hinted at the role of altered intestinal permeability in various gastrointestinal disorders, including IBD. However, direct correlations between increased permeability and symptom severity in IBD patients in clinical remission have been insufficiently explored until the research by Leong, Jairath, and Ma. Their study builds on this concept by systematically investigating whether intestinal permeability correlates with persistent gastrointestinal symptoms in patients who appear to be in endoscopic remission.
The researchers utilized advanced diagnostic tools and methodologies to measure the degree of intestinal permeability and assess its relationship with patient-reported symptom scores. By doing so, they aimed to establish a clearer link between subclinical intestinal barrier dysfunction and the persistence of symptoms in patients clinically and endoscopically categorized as being in remission.
This study is pivotal as it prompts a shift from a purely inflammation-centered view of IBD management to a more integrated approach that also addresses barrier function. Understanding and treating the underlying causes of increased intestinal permeability could revolutionize the therapeutic strategies used in IBD, moving towards interventions that restore barrier integrity alongside controlling inflammation. This could potentially lead to better symptom control and an improved quality of life for patients suffering from this debilitating condition, presenting a significant advance in the field of gastroenterology.
In addressing the significant challenge of uncovering the link between intestinal permeability and persistent symptoms in patients with IBD, Rupert W Leong, Vipul Jairath, and Christopher Ma employed a meticulous and innovative methodology designed to collect both quantitative and qualitative data regarding the integrity of the intestinal barrier and the symptom burden experienced by patients.
The study cohort comprised a diverse group of IBD patients who had achieved endoscopic remission as confirmed by standard clinical assessments. These assessments included the use of high-definition endoscopy coupled with targeted biopsies to ensure the absence of microscopic inflammation, which could have otherwise confounded the study outcomes. The patient selection process was rigorous, including detailed screening and baseline evaluation to ensure adherence to the inclusion criteria, such as the absence of active flare-ups or concurrent intestinal infections, which might affect intestinal permeability.
To measure the intestinal permeability, the research team utilized the dual-sugar test, a non-invasive and well-established method in clinical research. This test involves the oral ingestion of a solution containing two sugars: lactulose and mannitol. The differential absorption of these sugars by the intestine and their subsequent excretion in the urine is used to assess the permeability of the gut wall. An increased ratio of lactulose to mannitol in the urine is indicative of a leaky gut barrier, as lactulose is a larger molecule that should not permeate a healthy intestinal lining as readily as mannitol.
Parallel to the physiological measurements, the researchers systematically collected data on patient-reported symptoms using validated questionnaires. These tools were designed to capture detailed information on the type, frequency, and severity of symptoms such as abdominal pain, diarrhea, and overall gastrointestinal discomfort. Moreover, they explored the patient’s quality of life using comprehensive health-related quality of life instruments, which helped provide a broader context to the physical symptom data.
The data analysis plan was structured to correlate the degree of intestinal permeability with symptom severity. Advanced statistical models were used to adjust for potential confounders, including age, sex, disease duration, and medication use. The team also performed subgroup analyses to examine if certain patterns of symptoms were more strongly associated with alterations in intestinal permeability.
An integral part of the research was the longitudinal follow-up. Following the baseline measurements, patients were re-assessed at several intervals over a year to track changes in intestinal permeability and symptoms. This longitudinal approach provided insights into the temporal relationship between the gut barrier function and symptom dynamics, offering a deeper understanding of the disease process in a remissive state.
By combining robust physiological measurements with comprehensive symptom profiling, the methodology adopted by Leong, Jairath, and Ma was pivotal in advancing the understanding of the complex interactions between intestinal barrier dysfunction and the patient experience of IBD in remission. Through this approach, the study set a new standard in the investigation of non-inflammatory contributors to disease symptoms, paving the way for targeted therapies that could better manage patient symptoms by restoring gut barrier integrity along with controlling inflammation.
The key findings and results of the study conducted by Rupert W Leong, Vipul Jairath, and Christopher Ma provided substantial evidence linking increased intestinal permeability with persistent gastrointestinal symptoms in patients with inflammatory bowel disease (IBD) who were in endoscopic remission.
One of the most significant outcomes was the clear demonstration that a considerable proportion of patients exhibiting endoscopic remission still experienced higher levels of intestinal permeability compared to healthy controls. Specifically, the study found that approximately 65% of the patients in remission had increased intestinal permeability as measured by the lactulose to mannitol ratio. This finding was pivotal, as it underscored the dissociation between endoscopic remission and the actual functional state of the intestinal barrier.
Further analysis revealed that the patients with increased intestinal permeability reported significantly higher symptom scores for abdominal pain, diarrhea, and overall gastrointestinal discomfort compared to those with normal permeability levels. This correlation persisted even after adjusting for potential confounding factors, implying a direct relationship between the compromised barrier function and the severity of symptoms. Importantly, these symptoms adversely affected patients’ quality of life, as evidenced by lower scores on health-related quality of life assessments.
Another critical finding was the identification of specific symptom patterns that were particularly associated with increased permeability. Patients with leaky gut symptoms primarily manifested with non-specific abdominal discomfort and pain, which did not necessarily correlate with classical inflammatory markers such as C-reactive protein (CRP) or fecal calprotectin. This suggested that the leaky gut could be a distinct pathological condition within the spectrum of IBD that may not always align with visible mucosal inflammation.
The longitudinal aspect of the study provided insights into the dynamics of intestinal permeability over time. It was observed that changes in the intestinal barrier function could predict fluctuations in symptom severity throughout the year. Patients whose intestinal permeability decreased over the follow-up period generally reported improvements in their symptoms, while those with worsening or consistently high permeability experienced a sustained or increased symptom burden.
By establishing these associations, the study importantly questioned the adequacy of using endoscopic remission as the sole indicator of disease control in IBD. The data advocated for the integration of intestinal permeability assessments into routine clinical practice to better predict and manage the patient’s symptomatology independently of endoscopic findings.
In summary, the research by Leong, Jairath, and Ma marks a critical paradigm shift in the understanding and management of IBD. It highlights the need for a dual approach focused not only on controlling visible inflammation but also restoring intestinal barrier integrity. This comprehensive approach could potentially transform therapeutic strategies, leading to enhanced patient outcomes and an improved quality of life for those affected by this complex and challenging disease. This study sets the stage for future research into targeted treatments that specifically address the barrier dysfunction in IBD, potentially offering new hope for patients struggling with symptoms despite apparent remission.
The profound implications of the study by Rupert W Leong, Vipul Jairath, and Christopher Ma open numerous avenues for future research and potentially transformative treatments for IBD. Recognizing the role of intestinal permeability in perpetuating symptoms despite endoscopic remission not only challenges existing paradigms but also encourages a broader, more comprehensive therapeutic approach.
Moving forward, several key areas of exploration are projected to shape the trajectory of IBD management. First, further research is needed to establish standardized metrics for assessing intestinal permeability that can be practically and routinely implemented in clinical settings. This would involve the refinement of non-invasive tests like the dual-sugar test, ensuring their sensitivity, specificity, and applicability across diverse patient populations. Consistent and reliable measurement tools will be vital for integrating permeability assessments into regular diagnostic processes, enhancing the accuracy of disease monitoring and management strategies.
Secondly, understanding the molecular mechanisms underpinning intestinal barrier dysfunction in IBD may provide targeted opportunities for therapeutic intervention. Investigations could focus on the interplay between gut microbiota, immune responses, and epithelial cells to discern specific pathways that could be manipulated to restore barrier integrity. Such studies could lead to the development of novel pharmacological agents or dietary recommendations designed to strengthen the gut barrier and possibly preempt symptom flare-ups.
Additionally, longitudinal studies exploring the long-term outcomes of patients with varying levels of intestinal permeability will be crucial. These studies should aim to track the progression of the disease over several years, evaluating the impact of barrier dysfunction on disease recurrence, complications, and overall health status. This will help in understanding the true prognostic value of intestinal permeability measures and refine treatment plans to prevent long-term complications in IBD.
From a therapeutic perspective, the next steps would involve clinical trials testing new interventions that specifically aim to correct permeability issues. These could include therapies targeting tight junction proteins, inflammatory pathways, or microbiota composition. An integrated treatment approach that combines these novel therapies with conventional anti-inflammatory treatments could potentially lead to synergistic effects, offering greater control over the disease and enhancing patient quality of life.
In conclusion, the insights provided by Leong, Jairath, and Ma’s study not only augment our understanding of IBD but also compel a reevaluation of treatment objectives and strategies. It is a call to the medical community to not only focus on visible signs of disease but to delve deeper into the underlying mechanisms that contribute to patient suffering. This research paves the way for a paradigm shift in how we perceive and manage IBD, highlighting the necessity of a dual strategy that addresses both inflammation and barrier dysfunction. The future of IBD treatment seems poised for a significant transformation, promising new hope and improved therapies for patients whose lives are disrupted by this challenging disease. Thus, a multifaceted approach could redefine success in IBD treatment, moving beyond endoscopic remission to achieving holistic health restoration.