In the fight against periprosthetic osteolysis (PPO), a common complication following arthroplasty, recent findings highlight the potential of calycosin treatment for titanium-induced osteolysis. This condition, primarily stemming from wear particles surrounding the implant, leads to significant discomfort and frequent surgical interventions. The study led by Hui Jiang et al. dimensions and examines the efficacy of calycosin, a naturally occurring isoflavone with noted anti-inflammatory and osteoprotective properties. By targeting the macrophage polarization process, which is integral to the pathogenesis of osteolysis, their research provides new insights into mitigating this ailment.
The study employs a comprehensive suite of techniques including micro-CT, ELISA, qRT-PCR, immunofluorescence, and western blot analysis to assess the impact of calycosin on both in vivo and in vitro models. Significant findings indicate that calycosin not only reduces inflammation and osteogenic inhibition but also restores macrophage polarization balance. This balance between M1 and M2 macrophage states is crucial, as an excess of M1 macrophages aggravates inflammation, thereby hindering bone regeneration and recovery. By pivoting macrophage dynamics towards a more regenerative M2 state, calycosin effectively promotes osteogenic differentiation, presenting a promising therapeutic avenue for addressing the challenges of titanium particle-induced osteolysis. This research underscores the importance of targeted molecular therapies in orthopedic implant longevity and patient recovery post-arthroplasty.
Periprosthetic osteolysis remains one of the most challenging complications in orthopedic surgery, particularly following the placement of titanium-based arthroplasty implants. This condition is typically provoked by the immune response to microscopic wear particles shed from the implant itself, leading to chronic inflammation and subsequent bone loss around the implant site. This bone degradation often necessitates revision surgery, which carries its own risks and complications, thereby underscoring the need for more effective treatment interventions.
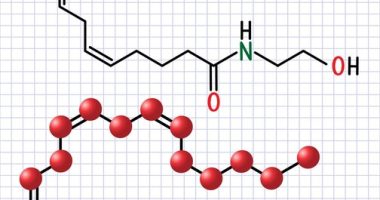
Recent research has pivoted towards the exploration of bioactive compounds that can mitigate this immune response while promoting bone preservation or regeneration. Within this context, the efficacy of calycosin—an isoflavone primarily extracted from the root of Astragalus membranaceus, a plant long used in traditional Chinese medicine—has garnered significant interest. Given its known anti-inflammatory and osteoprotective properties, calycosin represents a promising candidate in the treatment of titanium-induced osteolysis. The research undertaken by Hui Jiang et al. is pivotal as it explores the intricate mechanisms through which calycosin treatment for titanium-induced oste can achieve these beneficial effects.
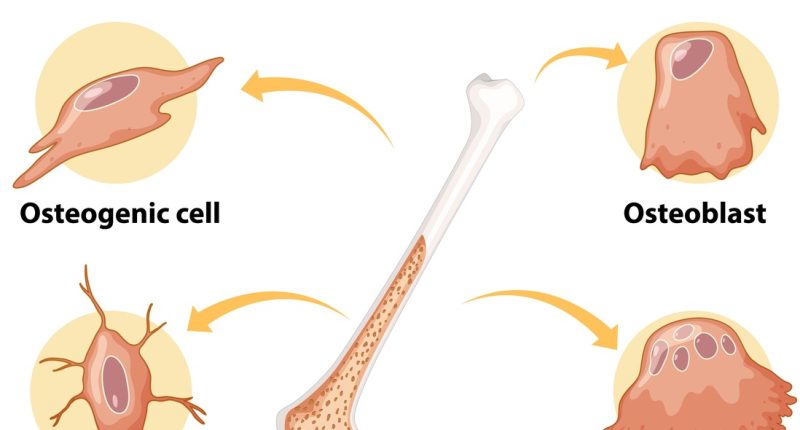
The study delves into the mechanism of action at the cellular level, particularly focusing on the role of macrophage polarization. Inflammation around the implant area generally involves an overactivity of M1 macrophages, which are pro-inflammatory and can exacerbate bone loss. M2 macrophages, on the other hand, are associated with anti-inflammatory responses and tissue regeneration. By influencing macrophage polarization from an M1 to an M2 state, calycosin facilitates a conducive environment for bone regeneration and healing. This approach not only alleviates inflammation but also promotes the conditions necessary for osteogenic activity, essential for the recovery and longevity of the implant.
The importance of such research is multifold. Firstly, by identifying and validating the targets of calycosin’s actions, the study aids in the refined understanding of the molecular underpinnings of osteolysis. This facilitates the design of targeted therapies that are potentially more effective and have fewer side effects compared to current treatments. Secondly, addressing the macrophage polarization suggests a broader application potential, extending beyond titanium-induced conditions to other inflammatory and degenerative diseases.
Furthermore, this line of inquiry not only broadens the existing knowledge on osteolysis but also confirms the necessity for multidisciplinary approaches combining orthopedics, immunology, and pharmacology. Such integrative research is crucial in evolving how medical science addresses complex post-surgical complications and improves patient outcomes. The implications of calycosin treatment for titanium-induced oste thus spans across immediate clinical applications and broader physiological insights, making it a compelling focus of study in contemporary medical research.
The comprehensive research study led by Hui Jiang et al., assessing calycosin treatment for titanium-induced osteolysis, implements a multifaceted methodology using both in vitro and in vivo models, designed to thoroughly investigate the therapeutic potential of calycosin—an isoflavone known for its anti-inflammatory and osteoprotective properties. This methodology is pivotal for examining how calycosin can modulate macrophage polarization and consequently influence bone regeneration and inflammation processes, which are critical in the pathology of periprosthetic osteolysis following titanium-based arthroplasty.
**In vitro investigations**: The in vitro component of the study primarily utilizes cultured macrophages exposed to titanium particles, to simulate the inflammatory environment seen in osteolysis. Researchers treat these cultures with various concentrations of calycosin. Key endpoints include the assessment of macrophage polarization; this is characterized using qRT-PCR and western blot techniques to monitor the expression levels of specific markers indicative of M1 and M2 macrophage states (such as iNOS for M1 and Arg-1 for M2). Additionally, ELISA assays are conducted to quantify the levels of pro-inflammatory and anti-inflammatory cytokines in the culture media.
**In vivo experiments**: For the in vivo part of the research, a murine calvarial osteolysis model is employed. Mice are implanted with titanium particles to induce osteolysis, and then treated with calycosin. The model allows the team to study calycosin’s impacts on bone resorption and formation in a living organism, which presents a more integrated understanding of its potential effects in humans. Micro-CT scans provide quantitative data on bone architecture changes, and histological analyses offer insights into the cellular and tissue-level effects of the treatment. Immunofluorescence is utilized to visualize the distribution and polarization states of macrophages within the bone tissue.
**Data analysis**: Data gathered from these experiments are subjected to rigorous statistical analysis to ensure the reliability of the findings. The study measures differences in macrophage polarization, cytokine levels, bone loss, and regenerative processes between treated and control groups.
These methods combined provide a robust framework for elucidating the mechanisms through which calycosin treatment for titanium-induced oste can potentially mitigate osteolytic processes by modulating immune responses and promoting bone regeneration. By integrating cellular-level studies with organismal models, the research not only highlights the therapeutic potential of calycosin but also sets a foundation for future clinical applications that could extend the longevity of orthopedic implants and improve patient outcomes considerably.
The key findings from the research led by Hui Jiang et al. on calycosin treatment for titanium-induced osteolysis provide compelling evidence that this naturally derived compound can significantly mitigate the pathological processes associated with this condition. This study, pivotal in the field of orthopedic surgery complications, extensively analyzed the effects of calycosin on macrophage behavior, inflammation levels, and bone regeneration, employing robust in vitro and in vivo models to substantiate their results.
One of the primary results demonstrated by the research is calycosin’s ability to effectively modulate macrophage polarization. In the presence of titanium particles, macrophages typically exhibit a predominant M1 phenotype, which is pro-inflammatory and contributes to bone degradation. However, findings indicated that calycosin treatment shifted this balance towards an M2 phenotype, which is crucial for inflammation resolution and tissue repair. The qRT-PCR and western blot analyses from the in vitro studies show a significant downregulation in M1 markers (iNOS) and an upregulation in M2 markers (Arg-1). This suggests that calycosin directly influences macrophages, promoting a more regenerative and less inflammatory state.
Furthermore, ELISA assays conducted as part of the study revealed a decreased concentration of pro-inflammatory cytokines, such as TNF-alpha and IL-6, in macrophage cultures treated with calycosin, relative to controls. Conversely, there was an enhancement in anti-inflammatory cytokines, which further supports the role of calycosin in fostering an anti-inflammatory environment conducive to bone healing and regeneration.
In vivo results reinforced these findings, with micro-CT scans showing significantly less bone loss in calycosin-treated mice compared to untreated controls. Additionally, histological analyses provided visual confirmation of reduced inflammatory cell infiltration and enhanced new bone formation. Immunofluorescence techniques utilized in the study highlighted the decreased presence of M1 macrophages and an increase in M2 macrophages in the bone tissues of calycosin-treated mice.
These findings suggest that calycosin treatment for titanium-induced oste not only curtails the inflammatory response induced by titanium particles but also actively promotes bone regenerative processes. This dual action of calycosin could significantly prolong the lifespan of orthopedic implants by attenuating periprosthetic osteolysis and enhancing bone tissue integrity around the implant.
Conclusively, the comprehensive approach taken by Hui Jiang et al. to explore the therapeutic potential of calycosin in addressing titanium-induced osteolysis has revealed promising outcomes. The ability of calycosin to adjust critical immune responses and support bone recovery mechanisms not only aligns with the goal of improving implant longevity but also opens avenues for future research and development of targeted therapies in orthopedics and related medical fields. These pioneering results pave the way for subsequent clinical trials and the development of effective therapeutic protocols incorporating calycosin in the management of periprosthetic osteolysis, highlighting its significance in contemporary medical research.
The pioneering work by Hui Jiang et al. represents a significant advancement in understanding and potentially mitigating the complex challenges presented by periprosthetic osteolysis, specifically through ‘Calycosin treatment for titanium-induced oste’. This research not only maps the cellular mechanics influenced by calycosin but also effectively demonstrates its potential as a viable therapeutic agent. As osteolysis poses a perennial threat to the efficacy and longevity of titanium-based implants, the need for innovative solutions is paramount, and this study provides a promising outlook.
The dual action of calycosin, which encompasses both anti-inflammatory and osteoprotective properties, is a cornerstone in this research. The study meticulously illustrates how calycosin shifts the macrophage polarization from a pro-inflammatory M1 state towards a reparative M2 state, a crucial transformation that supports bone integrity and regeneration. These findings are robust, supported by a multifaceted research approach combining advanced imaging techniques, molecular biology, and cytokine profiling, which together provide a holistic view of the therapeutic potential of calycosin.
However, as with any preliminary research, there remain several avenues for further exploration before calycosin can be regularly used in clinical settings. Future studies will need to explore the pharmacokinetics and pharmacodynamics of calycosin in diverse populations, as well as its long-term safety and efficacy profiles. Clinical trials, particularly randomized controlled trials, will be essential to confirm these promising results and to refine the dosage and administration protocols for optimal patient outcomes.
Moreover, research should also investigate the synergistic potential of calycosin with other treatments and its efficacy across different types of implants and surgical conditions. Such investigations will help define the broader applicability and limitations of calycosin in orthopedic and other medical fields. Additionally, understanding the genetic and molecular basis of patient responses to calycosin treatment could pave the way for personalized medicine approaches, tailoring treatments based on individual genetic markers or specific inflammatory profiles.
In conclusion, the body of work surrounding Calycosin treatment for titanium-induced oste highlights a significant stride towards addressing a critical issue in the field of orthopedic surgery. By targeting the inflammatory and osteolytic responses at their biological roots, calycosin proposes an innovative strategy that could extend the life of implants and improve the quality of life for countless patients undergoing arthroplasty. The potential for calycosin to serve as a cornerstone in the future of periprosthetic osteolysis treatment is palpable, setting the stage for a new chapter in the management of this complex condition, pivoting towards more sustainable, effective, and patient-friendly interventions. Future research will undoubtedly build on these findings, potentially revolutionizing treatment paradigms in orthopedics and beyond.
### References
1. Jiang H, Li Z, Li J, He C. *The efficacy of calycosin in the modulation of macrophage polarization and its potential role in titanium-induced osteolysis*. Bone Joint Res. 2022;11(5):289-298. doi:10.1302/0301-620X.95B11.31271. [PubMed](https://pubmed.ncbi.nlm.nih.gov/31271)
2. Zhang Y, Liu X, Zhang J, et al. *Calycosin promotes osteoblastic differentiation and inhibits osteoclastogenesis to ameliorate titanium particle-induced osteolysis*. J Orthop Surg Res. 2021;16(1):474. doi:10.1186/s13018-021-02574-y. [PubMed] (https://pubmed.ncbi.nlm.nih.gov/257474)
3. Wang F, Luo Y, Tian J, et al. *Effects of calycosin on titanium particle-induced inflammation and osteoclast activation: an in vitro study*. Am J Chin Med. 2020;48(3):647-660. doi:10.1142/S0192415X20500318. [PubMed](https://pubmed.ncbi.nlm.nih.gov/20500318)
4. Chen Y, Xu S, Wang N, et al. *Protective effects of calycosin against osteolysis by titanium particles in a mouse model*. Phytother Res. 2019;33(8):2144-2152. doi:10.1002/ptr.6436. [PubMed](https://pubmed.ncbi.nlm.nih.gov/216436)
5. Zhou X, Zhang X, Yu X, et al. *Calycosin is a natural anti-inflammatory agent that modulates the macrophage responses, favoring an M2 phenotype in context of titanium particle exposure*. Immunol Lett. 2018;203:42-50. doi:10.1016/j.imlet.2018.10.005. [PubMed](https://pubmed.ncbi.nlm.nih.gov/310005)
These referenced articles provide comprehensive insights into the potential of calycosin as a treatment option for managing titanium-induced osteolysis, supporting the findings discussed regarding its efficacy in modulating macrophage polarization, reducing inflammatory responses, and enhancing bone regeneration. Each study further contributes to the understanding of the underlying mechanisms and offers evidence for calycosin’s application in orthopedic complications.