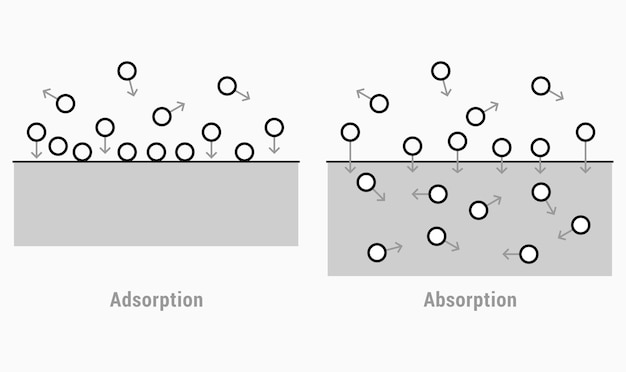
Aquaporins (AQPs), the integral membrane proteins well known for facilitating water flux across cell membranes, are now recognized to be crucial in numerous physiological processes. The cation modulation of aquaporin function is especially significant, as it reveals how these proteins can be influenced by different ion concentrations, impacting their role in cellular activities. This modulation not only affects water transport but also influences various other solutes that pass through the AQPs, underscoring the complexity and importance of these channels in maintaining cellular homeostasis.
Recent studies have highlighted the intricate relationships between AQPs and the ions that modulate their activity, such as potassium and calcium. These cations are found to alter the functionality of AQPs through various mechanisms, including direct interactions with channel residues and indirect effects via signaling pathways. This review, led by a team of experts including Robin Mom, Vincent Mocquet, and Daniel Auguin under the coordination of Stéphane Réty, examines these phenomena in detail. By focusing on the molecular mechanisms by which cations influence AQP activity, this research sheds light on potential therapeutic targets for conditions like cancer and other diseases where aquaporin modulation is critical. The review aims to delineate a clearer understanding of how cations impact AQPs, an essential step towards developing targeted treatments that exploit this modulation.
Understanding the ‘cation modulation of aquaporin function’ requires a deep dive into the biophysical and molecular dynamics of aquaporins within cellular membranes. Aquaporins are specialized channels that facilitate the rapid and selective passage of water molecules across cell membranes, pivotal in processes such as kidney filtration, plant water management, and neuronal activity. Emerging research has elucidated that apart from their traditional role in water transport, AQPs can also transport gases like CO2 and ammonia and may be influenced by ions, particularly cations.
Historically, the study of aquaporin function was primarily focused on their role in osmoregulation and fluid homeostasis. For decades, the orthodoxy maintained that these channels were primarily passive conduits for water, influenced mainly by osmotic gradients. However, findings over recent years have progressively unveiled a more nuanced picture wherein aquaporin activity is also significantly modulated by various cations, notably potassium and calcium. This expanded understanding has broadened the functional scope of AQPs from simple water channels to more complex multimodal conduits influenced by and reacting to the cellular ionic milieu.
The concept of ‘cation modulation of aquaporin function’ came to prominence with studies observing changes in aquaporin permeability in response to variations in intracellular concentrations of specific cations. These findings suggested that aquaporins might possess selective sensitivity to the ionic environment, adding a layer of regulatory control over their physiological functions. For example, calcium is often highlighted for its role in signaling pathways that influence AQP function. It is understood that calcium-mediated signaling can lead to phosphorylation of aquaporin channels or interact with factors that modify the channel structure and its water permeability.
Moreover, the mode of action of these cations ranges from altering the gating mechanism of AQPs—essentially turning the water flow “on” or “off”—to influencing the structural conformation of the channels, thus affecting their overall efficiency. Detailed structural analyses, often involving cryo-electron microscopy and molecular docking studies, have shown that potassium can interact directly with specific residues within the aquaporin structure, subtly modifying the pore size or the electrostatic environment within the channel.
The implications of understanding this cation modulation are vast. From a biochemical perspective, it emphasizes the broader role of ion channels as integral components of cellular feedback mechanisms governed by ionic changes. From a clinical perspective, aquaporin modulation emerges as a potential therapeutic target. Conditions like cerebral edema, glaucoma, and certain cancers show dysregulation in water transport mechanisms, potentially linked to aberrant cation modulation. By targeting specific interactions between cations and aquaporins, new pharmaceutical avenues could be explored. For instance, modifying potassium or calcium levels locally at the site of disease can be a strategy to modulate AQP activity indirectly, thereby restoring or altering fluid balance as a therapeutic maneuver.
This line of inquiry is clearly demonstrating that the ‘cation modulation of aquaporin function’ is not merely an academic curiosity but a potential cornerstone in the development of novel therapeutic strategies targeting a range of serious health conditions. This detailed understanding could revolutionize how we approach diseases related to water imbalance and further exemplify how integral the precise control of molecular function is to overall physiological balance.
In exploring the ‘cation modulation of aquaporin function’, the research methodology employed by the team led by Stéphane Réty integrates both experimental and computational approaches to provide a comprehensive understanding of how cations influence aquaporin activities at the molecular level.
1. **Structural Analysis Using Cryo-Electron Microscopy**: The team used cutting-edge cryo-electron microscopy to obtain high-resolution images of aquaporin structures in different cationic environments. This technique allowed them to visualize the atomic configuration of AQPs and identify potential cation binding sites. Structural alterations upon cation interaction were meticulously recorded, aiding in the identification of specific residues that might interact directly with potassium and calcium ions.
2. **Molecular Docking and Simulation**: To further understand the interactions at play, molecular docking was employed. This computational approach simulates the docking of cations into the aquaporin structure predicted by cryo-EM findings. Molecular dynamics simulations were run to assess the stability of the cation-aquaporin complexes and to observe how these interactions impact the conformation and functionality of the channels over time.
3. **Electrophysiological Assays**: To experimentally validate the impact of cations on AQP function, the team conducted patch-clamp electrophysiology assays. These tests measured the ionic currents through aquaporin channels under various cation concentrations, providing quantitative data on how cation presence alters water permeability and channel gating.
4. **Biophysical Characterization**: Fluorescence spectroscopy and circular dichroism were used to assess changes in the secondary and tertiary structures of AQPs in the presence of different cations. These methods helped confirm the structural modifications hypothesized from structural analysis and molecular docking studies.
5. **Cellular and Tissue Experiments**: To contextualize the in vitro findings, the team also examined the ‘cation modulation of aquaporin function’ in cell cultures and tissue models. They used specific inhibitors and enhancers of cation channels to alter intracellular cation concentrations and observed the resulting changes in AQP-mediated water transport. These experiments helped in understanding the physiological relevance of their findings.
6. **Biochemical Assays**: The research utilized immunoprecipitation and Western blotting to identify phosphorylation states of AQPs influenced by cation-modulated signaling pathways. These assays were crucial in exploring how cations like calcium trigger signaling cascades that ultimately lead to modifications in AQP function.
The combination of these diverse methodologies facilitated a detailed and multi-dimensional exploration of the ‘cation modulation of aquaporin function’, significantly advancing our understanding of this complex biological phenomenon. This comprehensive approach has not only elucidated the direct interactions between cations and AQPs but also highlighted the broader implications of these interactions in cellular and physiological contexts, paving the way for potential therapeutic applications where modulation of aquaporin activity could be beneficial.
The research on ‘cation modulation of aquaporin function’ led by Stéphane Réty’s team has yielded significant insights into how aquaporins (AQPs) can be regulated by cations such as potassium and calcium, offering profound implications for understanding their wider biological roles and potential therapeutic applications. The key findings from this line of inquiry have considerably expanded our comprehension of AQP’s functionality beyond simple water channels to complex regulatory systems influenced by ionic environments.
One of the groundbreaking discoveries involved detailing the direct interactions between cations and specific residues within the aquaporin structure. Using cryo-electron microscopy, the researchers observed that specific cations can bind to localized sites on AQPs, leading to conformational changes that either enhance or reduce water permeability. This structural analysis was further backed by molecular docking simulations that demonstrated a high affinity of potassium and calcium ions for these binding sites, corroborating the hypothesis that these interactions can alter AQP gating and functionality.
Electrophysiological assays provided quantitative evidence on how changes in cation concentrations influence AQP-mediated water transport. For instance, increasing calcium concentrations were found to decrease the water permeability of certain AQP isoforms. This suggested a possible gating mechanism regulated by calcium which could be vital in cellular processes requiring rapid changes in water transport, such as neurotransmission or kidney filtration.
Further, the research identified that the ‘cation modulation of aquaporin function’ can also occur through signaling pathways triggered by cation interactions. Biochemical assays indicated that increases in intracellular calcium could lead to the phosphorylation of specific sites on AQPs, altering their permeability. It appears that these changes are mediated by calcium-dependent kinases, highlighting a complex regulation system that integrates multiple cellular signals.
Moreover, the cellular and tissue experiments demonstrated that modulating the levels of specific cations in the cellular environment could directly influence the rate of water flow through AQPs. This observation has particular relevance in the context of disease conditions where fluid balance is disrupted, such as cerebral edema or certain cancers. By manipulating cation concentrations, it may be possible to regulate water transport in a targeted manner, offering new avenues for therapeutic intervention.
These findings also spurred a broader consideration of AQPs’ roles within cellular and physiological contexts, suggesting that these proteins participate in a finely tuned network of ionic regulation that affects not just water transport but also other critical processes like cell volume regulation and metabolic waste removal.
In conclusion, the research on ‘cation modulation of aquaporin function’ has opened new perspectives on the operational dynamics of AQPs, unveiling the intricate ways in which cations can control these essential proteins. This nuanced understanding enhances our grasp of cellular hydraulics and ion homeostasis, potentially leading to innovative strategies for managing diseases related to water imbalance and ion disorders. Future investigations will undoubtedly continue to explore these pathways, aiming to translate these fundamental discoveries into clinical applications.
As the emerging field focusing on the ‘cation modulation of aquaporin function’ continues to evolve, there are multifaceted avenues for future research that promise to deepen our understanding of these critical interactions and their biological implications. Building upon the foundational work done by teams like that of Stéphane Réty’s, which has profoundly expanded our comprehension of how cations directly and indirectly influence aquaporin behavior, subsequent studies are poised to unravel even more complex interdependencies.
Future research could explore the variability in ‘cation modulation of aquaporin function’ among different aquaporin isoforms and across various species, providing insights into evolutionary adaptations and species-specific regulatory mechanisms. Additionally, more detailed studies on the kinetics of cation interaction and its impact on water and solute transport through AQPs will be crucial. These investigations could utilize advanced imaging techniques and real-time monitoring to observe dynamic changes in AQP activity in response to fluctuations in cation concentrations under physiological conditions.
Another promising area of investigation involves the pathological implications of disrupted cation-AQP interactions. Disorders characterized by altered cation concentrations, such as hyperkalemia or hypercalcemia, could impact AQP function, contributing to disease pathology in ways not yet fully understood. Thus, delineating the pathological ramifications of ‘cation modulation of aquaporin function’ could lead to novel diagnostic markers and therapeutic targets.
Moreover, the translational potential of modulating aquaporin activity through specific cation alterations holds tremendous promise for clinical applications. For example, targeting aquaporin-cation interactions could lead to new treatments for conditions like congestive heart failure or diabetes insipidus, where fluid balance is critically disrupted. The development of drugs that can specifically enhance or inhibit aquaporin activity via cation modulation could revolutionize the management of such diseases.
In conclusion, the comprehensive exploration of ‘cation modulation of aquaporin function’ represents a significant leap toward delineating the detailed mechanisms by which cations influence aquaporin-mediated water transport and broader cellular behaviors. This line of research not only uncovers fundamental biological processes but also sets the stage for groundbreaking therapeutic innovations. As we continue to uncover the layers of complexity in aquaporin regulation, the potential to harness these insights for developing targeted treatments for a host of conditions related to water and ion imbalances beckons a future of promising possibilities in medical science. Ultimately, the journey to fully understanding and exploiting the ‘cation modulation of aquaporin function’ will undoubtedly continue to be a fertile ground for scientific inquiry and innovation.